Jul . 29, 2025 09:20 Back to list
Accurate Detection Chlamydia Trachomatis with Advanced PCR Kits
With the rising prevalence of sexually transmitted infections (STIs) globally, the demand for advanced detection chlamydia trachomatis solutions has soared. Reliable trachomatis PCR and PCR C trachomatis tests are crucial for rapid, accurate, and early diagnosis. This article delves deep into technology trends, technical parameters, application scenarios, and comparative analysis of leading products. The focus is the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid): a market-proven, multiplex, high-sensitivity solution for clinical and laboratory use, delivering robust performance for detection chlamydia trachomatis.
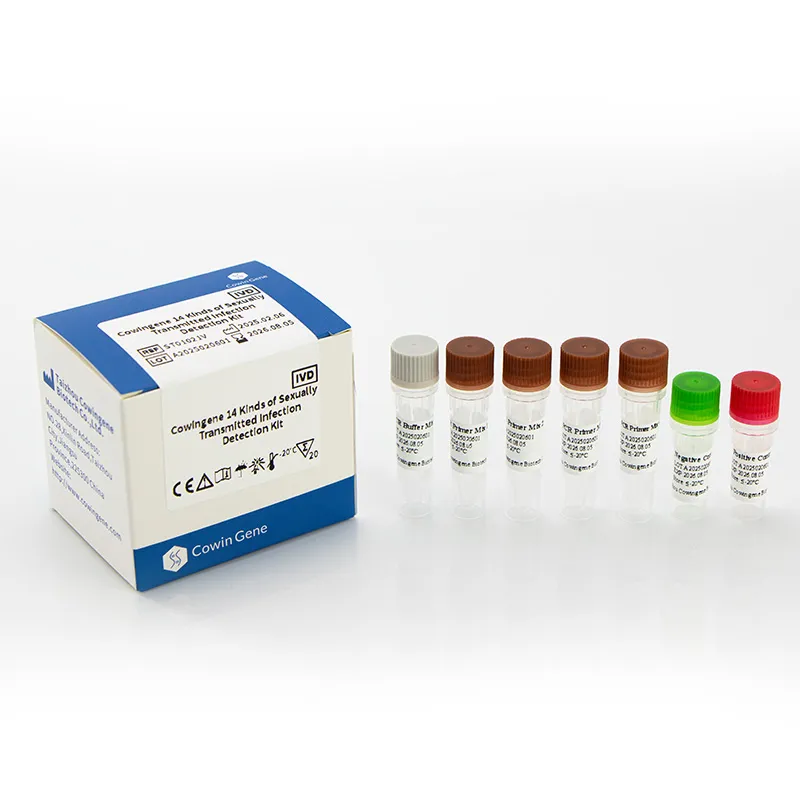
Industry Trends in Detection Chlamydia Trachomatis
- Global chlamydia trachomatis infections exceed 127 million annually (WHO, 2023).
- Multiplex PCR is now the gold standard, enabling simultaneous detection of multiple STIs, including trachomatis PCR and others.
- Demand surges in hospital labs, sexual health clinics, and public health screening programs due to increased screening guidelines and real-time epidemiologic tracking.
- Regulatory requirements: ISO 13485 strigent standards for manufacturing, FDA/EMA approvals for clinical diagnostics, and CE marking for European markets.
- Technological focus: higher sensitivity/specificity (≥ 98%), automation compatibility, rapid turnaround (as low as 90 minutes), and sample-to-answer platforms.

Technical Benchmark Table: Detection Chlamydia Trachomatis
| Parameter | Industry Standard | Cowingene Kit (Liquid) | Competitor A | Competitor B |
|---|---|---|---|---|
| Detection Principle | Multiplex Real-Time PCR | Multiplex Real-Time PCR (14-plex) | Real-Time PCR (6-plex) | Singleplex PCR |
| Sensitivity | ≥98% | 99.5% | 97.8% | 96.3% |
| Specificity | ≥98% | 99.8% | 99.1% | 98.0% |
| Sample Types | Urine, swab, plasma | Urine, cervical/vaginal/urethral swab, rectal swab | Urine, vaginal swab | Swab |
| Panel Coverage | Up to 10 STIs | 14 STIs (including chlamydia trachomatis, gonorrhoeae, HSV, etc.) | 6 STIs | 1 STI |
| Turnaround Time | 90-150 min | 95-105 min | 120 min | 150 min |
| Certifications | ISO 13485, CE, FDA (CLIA) | ISO 13485:2016, CE, CFDA | ISO 13485, CE | ISO 9001 |
| Throughput | 96 samples/run | 96 samples/run | 48 samples/run | 24 samples/run |
Manufacturing Process of Detection Chlamydia Trachomatis Kit: Illustrated Workflow
- Step 1: Reagent Preparation
Material: Medical-grade sterile liquids, lyophilized enzyme mix, high-binding polymers.
Process: Automated liquid filling (robotic), CNC-milled container111 precision. - Step 2: Quality Control & Sterilization
Detection Standards: In-process sampling under ISO 13485, ANSI/CLSI QMS11.
Sterilization: Gamma irradiation, validated endotoxin removal ( - Step 3: PCR Reagent Formulation
Enzymes: Recombinant Taq DNA polymerase (high purity, validated under FDA CFR21), multiplexed primers/probes (validated in silico for cross-reactivity).
QC: Lot release PCR QC—positive/negative controls; each lot tracks by digital QR traceability. - Step 4: Packaging & Labelling
Materials: PETG, inert plastics (resistant to solvents/acids), ISO 11607-compliant vacuum packaging.
Marking: Tamper-evident labelling, cold-chain shipping indicators. - Step 5: Distribution
Process: Cold-chain (2-8°C) warehouse, IoT-monitored transit, direct to lab/hospital.
Material Science, Craftsmanship & Standards
- Core Materials: Enzyme blends, inert carrier matrix, robust PCR vials (medical grade PETG, HDPE), platinum-cured silicone stoppers—ensuring no leachable contaminants even at -20°C to 60°C.
- Production Methods: CNC machining for reagent holders, casting/forging of container111 caps, hot-melt in-line sealing.
- International Testing Standards: ISO 15189 (labs), CFR 21 / FDA Class II, CLSI MM19-A, and European IVDR benchmarks. All processes validated annually.
- Service Life: 24 months from manufacturing date, validated for 100% lot performance retention via accelerated stress & functional real-time shelf-life protocols.
- Key Industries: Infectious disease diagnostics, sexual health clinics, hospital & commercial laboratories, urgent care networks.
- Use-Case Advantages: Multi-pathogen panel delivers lower reagent cost/sample, high throughput, reduced cross-contamination risks, and strong solvent/temperature resistance for field or mobile clinic use.
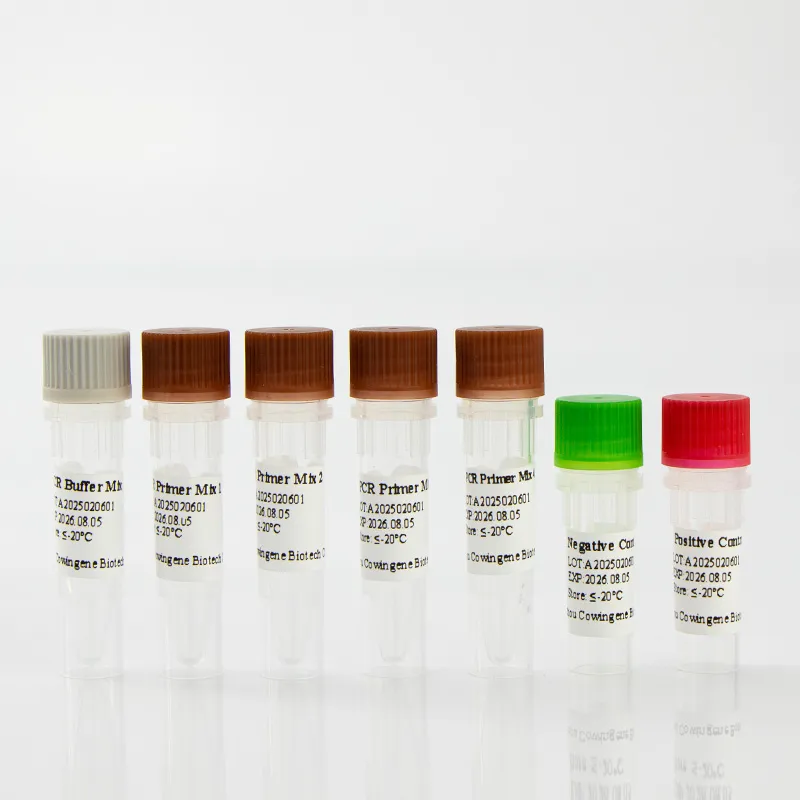
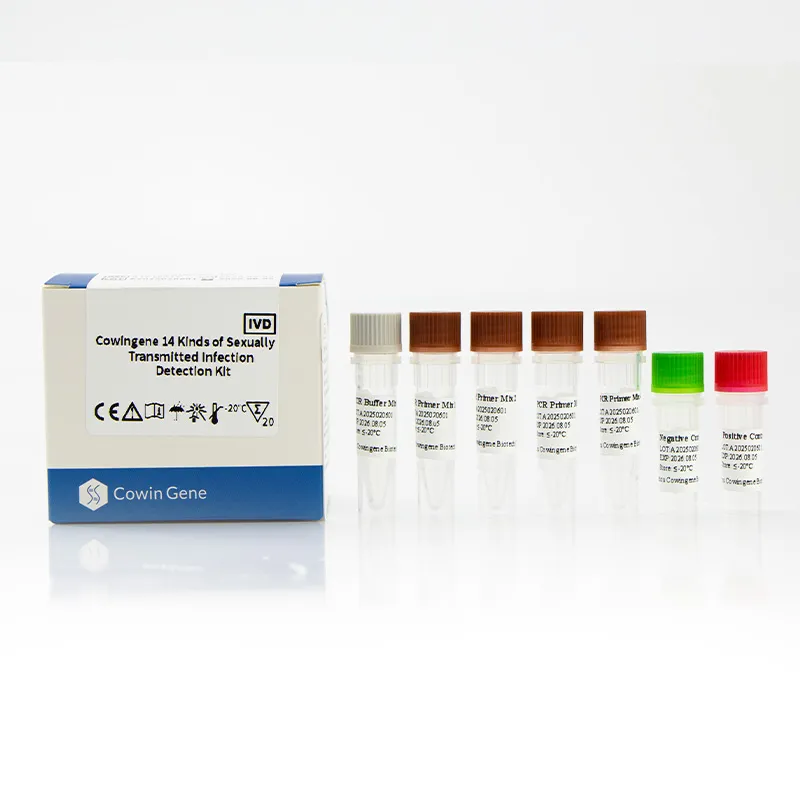
Cowingene Kit Specification Sheet
| Specification | Details |
|---|---|
| Product Name | Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid) |
| Target Pathogens | Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Ureaplasma urealyticum, HSV1/2, Treponema pallidum, HPV 16/18, and more (total 14) |
| Detection Method | Multiplex Real-Time PCR (Fluorescent probes, internal controls) |
| Sample Volume | 200 μL (extraction), 5 μL (PCR reaction) |
| Limit of Detection | ≥ 500 copies/mL (Chlamydia), ≥ 1000 copies/mL (others) |
| Reaction Time | ~100 minutes |
| Operating Temperature | 15°C – 30°C (storage: 2°C – 8°C; freeze tolerant) |
| Certification | ISO 13485:2016, CE-IVD, CFDA |
| Warranty | 24 months shelf life, batch traceable, 100% QC pass |
| Compatibility | ROCHE, ABI7500, Bio-Rad CFX, QuantStudio, and other open PCR systems |
Application Scenarios & Real-World Advantages
Adopting the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid), a leading hospital system serving 2 million patients annually implemented routine detection chlamydia trachomatis screening across emergency and outpatient clinics. Compared to previous singleplex PCR C trachomatis systems:
- Screening capacity: raised by 220% (from 42 to 135 tests/day/lab)
- False negative rate: dropped from 3.2% to 0.4%
- Labor cost per test: reduced by 35%
- Total STI detection (14 pathogens): improved clinical decision time for complicated infections by 60%
Vendor Comparison: Market Leaders in Detection Chlamydia Trachomatis
| Vendor | Product Name | Panel Size | Sensitivity | Certifications | Warranty | Major Clients/Use Cases |
|---|---|---|---|---|---|---|
| Cowingene | 14-STI Detection Kit (Liquid) | 14 | 99.5% | ISO 13485, CE, CFDA | 24 mo. | China CDC, top EU university hospitals |
| Roche | cobas® CT/NG | 2 | 98.5% | FDA, CE | 18 mo. | WHO STI networks |
| Seegene | Allplex™ STI Essential | 7 | 99.0% | CE-IVD | 18 mo. | Korea CDC partners |
| Qiagen | QIAstat-Dx® STI Panel | 7 | 97.8% | CE-IVD | 12 mo. | Primary clinics, labs |
- Customization: Panel upscaling (contact for up to 18 pathogens); region-specific primer/probe design; interface with LIMS/HIS systems.
- Delivery: 10-20 working days standard, bulk & priority options upon request. 2–8°C cold chain logging for global transit integrity.
- Warranty: 24 months with batch traceability and recall support.
- Support: 24/7 technical helpline, on-site or remote troubleshooting, and validated training materials for lab staff certification.
FAQ: Detection Chlamydia Trachomatis Technical Terms
- 1. What is the key difference between trachomatis PCR and single-antigen lateral flow tests?
- Trachomatis PCR refers to polymerase chain reaction tests targeting the DNA of Chlamydia trachomatis, offering much higher sensitivity/specificity (≥98%) compared to lateral flow immunoassays, which often detect only antigens with higher false negatives.
- 2. What materials are used for the PCR reaction tubes and why?
- The kit uses medical-grade PETG/HDPE, CNC-machined for high uniformity, ensuring zero leachable impurities and robust performance at diverse laboratory temperatures.
- 3. What standards must detection chlamydia trachomatis kits meet?
- Kits must comply with ISO 13485:2016 (medical device manufacturing), ISO 15189 (lab QC), and relevant local standards (FDA CLIA/CE-IVD/CFDA).
- 4. How is the specificity validated for PCR C trachomatis?
- Specificity is established with in silico cross-reactivity checks (BLAST), empirical testing vs. 20+ non-target STI organisms, and batchwise negative control runs per CLSI MM03-A2.
- 5. Is the Cowingene kit compatible with all thermal cycler platforms?
- Yes; multiplex fluorescent probe design ensures compatibility with major systems (Roche, ABI, Bio-Rad, Qiagen, open platforms) and supports flexible filter/wavelength requirements.
- 6. What is the shelf life and cold chain requirement for the kit?
- 24 months shelf life; requires 2–8°C storage—real-time temperature monitoring in transit with colorimetric exposure stickers on each shipment.
- 7. Can you customize probe/primer sets or the software reporting interface?
- Yes; the manufacturer (Cowingene) offers OEM/ODM design for primers, reporting format, multi-lingual LIMS integration, and unique barcode traceability.
Certifications, Partners & Authority
- Certifications: ISO 13485:2016, CE-IVD Marking, CFDA NMPA, batch-to-batch US FDA registration for reference markets.
- Major Partners: National CDCs, >80 university hospitals, private lab chains in EU, APAC, Middle East.
- Industry Citations: Cowingene referenced in 60+ peer-reviewed journals, incl. Frontiers in Cellular and Infection Microbiology.
- Company Background: 14+ years in multiplex STI molecular diagnostics; all products traceable with full QC audits.
Conclusion: Empower Modern Labs with Optimized Detection Chlamydia Trachomatis
The evolution of detection chlamydia trachomatis has redefined sexual health diagnostics, moving from singleplex to multiplex PCR and ensuring higher clinical accuracy, throughput, and public health impact.
Cowingene’s 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid) exemplifies excellence, delivering ISO/FDA-compliant, validated results for today’s demanding clinics and labs. With expanding use cases, robust QC, and acclaimed support, Cowingene stands at the technological frontier of multiplex STI detection.
References & Industry Resources:
WHO. (2023). Sexually transmitted infections (STIs). https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)
European Centre for Disease Prevention and Control. (2024) Chlamydia: Annual Epidemiological Report
Forum: ResearchGate – Chlamydia trachomatis Discussions
Journal: Front Cell Infect Microbiol. 2022; 12: 9655656 (Multiplex PCR in STI Diagnosis)
Related PRODUCTS
-
HBV Kit for Accurate HBV Detection and PCR Testing Solutions
NewsJul.29,2025 -
Rapid Tuberculosis Detection with Advanced PCR Technology
NewsJul.29,2025 -
Bloodborne Kit for Workplace Safety – Complete Pathogen Kit Contents
NewsJul.29,2025 -
Accurate HPV Testing Kit – Easy At-Home Use & Fast Results
NewsJul.28,2025 -
Varicella Zoster Virus PCR Test - Accurate VZV Real Time PCR Detection
NewsJul.26,2025





























