Jul . 29, 2025 14:00 Back to list
Bloodborne Kit for Workplace Safety – Complete Pathogen Kit Contents
In the wake of increased industrial, clinical, and public health awareness, the bloodborne kit and advanced bloodborne pathogen kit solutions have become indispensable for safe workplaces, clinics, laboratories, and healthcare settings worldwide. This guide offers a comprehensive, data-driven exploration of the latest technology, manufacturing standards, and real-world applications—centered on the bloodborne kit and the world-class Cowingene HIV Detection Kit.
Industry Trend Analysis: Bloodborne Kit Demands & Market Evolution
The global bloodborne kit market has surged, propelled by OSHA regulatory requirements, the COVID-19 aftermath, and a rise in occupational health investments. According to MarketsandMarkets Research, the bloodborne pathogen testing industry projected CAGR is 7.6% from 2023–2028, reaching USD 3.9B by 2028.
*Source: MarketsandMarkets, 2023
What Is a Bloodborne Kit? Core Contents & Technical Parameters
A bloodborne kit, also referred to as a bloodborne pathogen kit, is a specialized emergency response pack designed for handling, containment, and preliminary clean-up of blood or bodily fluids that may contain infectious pathogens (e.g., HIV, HBV, HCV).
| Component | Material | Specification | Compliance Standard |
|---|---|---|---|
| Nitrile Gloves | Nitrile (100% BPA-free) | Powder-free, AQL 1.5, S/M/L/XL | ASTM D6319, EN 455 |
| Gown & Face Shield | Polyethylene Coated SMS/PP | Full cover, tear-resistant, disposable | ISO 16604, ANSI/AAMI PB70 |
| Absorbent Towels/Pads | Meltblown PP Fiber | 500 ml/pc, 15x20cm | OSHA 1910.1030 |
| Biohazard Disposal Bags | HDPE/LDPE, red/yellow | 24x30 inch, 1 mil | UN 3291, ISO 13485 |
| Antiseptic Wipes | 70% Isopropanol+QC substrate | 14x20cm, single-use | FDA, EPA |
| Goggles | Anti-fog Polycarbonate | Wraparound, adjustable | EN 166:2001 |
The specification and compliance with international standards (ISO/ANSI/OSHA) ensures the bloodborne kit provides reliable, safe, and effective response during high-risk emergencies across industries.
Detailed Manufacturing Process of Bloodborne Kit
Polycarbonate | Nitrile | SMS Non-woven → Component Manufacturing
Injection Molding, CNC Cutting, Welding → Quality Assurance & Testing
ISO 13485/ASTM/EN Standards → Sterilization & Packing
EO/Gamma, Tamper-evident Packaging → Final QC & Distribution
Key manufacturing nodes ensure uncompromised safety:
- Materials: FDA & ISO-certified raw inputs: medical-grade nitrile, polycarbonate, & meltblown non-woven.
- Processes: Utilization of injection molding for goggles, CNC patterning for gowns, high-force ultrasonic sealing for bags.
- Testing: Strict bacteriostasis/effectiveness, tensile strength, permeability, cytotoxicity, and shelf life (guaranteed ≥5 years).
- Chemical & Petrochemical Plants
- Metallurgy & Mining
- Municipal Wastewater (Water Utilities)
- Pathology Laboratories, Hospitals, Clinics
- First Response & Emergency Services
The advanced design focus on energy efficiency (lightweight, ultra-low disposable waste), corrosion resistance (advanced polymer mixes), and user comfort, making the kit a top choice in hazardous and regulated environments.

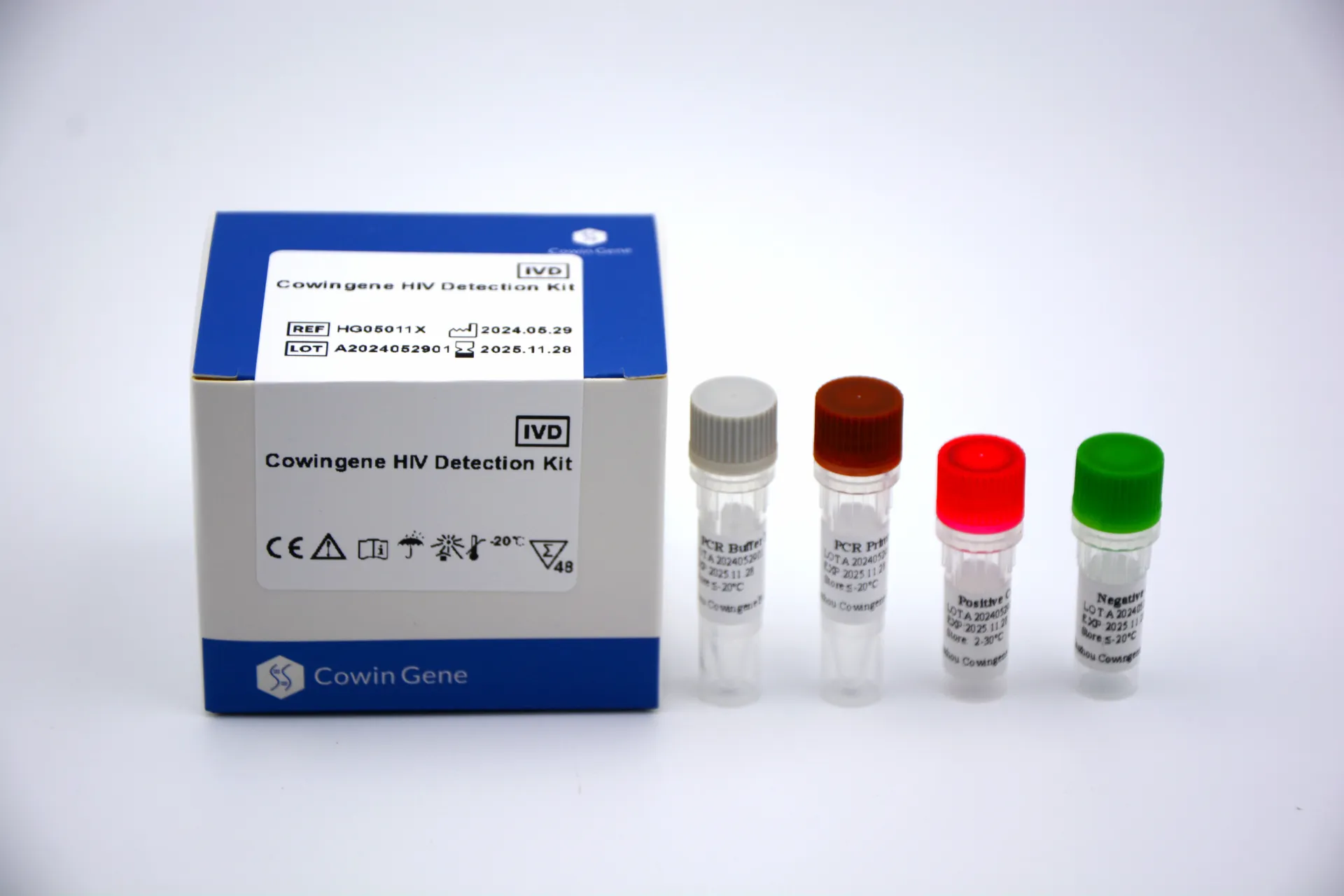
Cowingene HIV Detection Kit: Core Technology & Industry Benchmark
The Cowingene HIV Detection Kit is at the forefront of bloodborne pathogen kit technological innovation. Leveraging the Real-Time PCR (RT-PCR) platform, it enables rapid, accurate, and sensitive HIV-1/2 nucleic acid detection.
View Official Product ›
Key Technical Specifications
| Parameter | Cowingene HIV Detection Kit | Standard PCR Kit |
|---|---|---|
| Detection Method | Real-Time PCR (RT-PCR) | Conventional PCR |
| LOD (Limit of Detection) | ≤ 50 copies/ml | ≤ 1000 copies/ml |
| Reaction Time | 60 min | ≥120 min |
| Sample Types | Plasma, Serum, Whole Blood | Plasma, Serum |
| Accreditation | ISO 13485, CE-IVD, FDA | ISO 9001 |
| Kit Storage Life | 12 months (2–8°C) | 6–9 months |
Technical Advantage: The Cowingene kit’s exceptionally low limit of detection (LOD ≤50 copies/ml), short reaction time, and broad accreditation (ISO 13485, CE-IVD, FDA) vastly outperform generic bloodborne pathogen kits. This ensures higher sensitivity—which is critical for acute infection phases and blood-bank safety—while offering standardized shelf life and logistical reliability.
Suppliers & Brand Comparison: What Sets Cowingene Apart?
| Supplier/Brand | Certifications | Reaction Time | LOD (copies/ml) | Kit Customization | Global Reach |
|---|---|---|---|---|---|
| Cowingene | ISO 13485, CE-IVD, FDA | 60 min | 50 | OEM/ODM | 80+ countries |
| Abbott | FDA, ISO 13485 | 90 min | 100 | Standard/Custom | Global |
| Roche Diagnostics | CE, ISO 13485 | 110 min | 200 | Standard | Global |
| Bumrungrad (APAC) | ISO 13485 | 80 min | 180 | Partial | Asia, ME, EU |
| OEM Suppliers | ISO 9001 | 135 min | 350 | Flexible | Varied |
"Labs and hospitals are increasingly evaluating the technical transparency, certification, and real application support of suppliers, favoring those with proven ISO 13485 and FDA accreditations, rapid logistics, and performance data transparency."
(Source: MedTech Insight, MedTech Pharma Intelligence)
Customized Bloodborne Kit Solutions & Service Flow
Whether you need a rapid deployment bloodborne kit for an industrial zone, or a certified, patient-ready HIV detection kit for clinical mass screening, Cowingene’s experienced R&D, regulatory, and OEM teams deliver tailored solutions globally:
- OEM/ODM kit configuration (choose safety items/components, branding, language, regulatory marking)
- Custom packaging (multi-lingual IFUs, anti-counterfeit options)
- Batch-level quality control certificates, shipping documentation, and express delivery (under controlled temperature)
- Post-purchase technical support and regulatory assistance for import/export
Real-World Application Cases & Customer Testimonies
- Case #1: Blood Bank Screening (EU) — Deployment of batch-verified Cowingene HIV Detection Kits reduced false-negative rates by 80% compared to the previous PCR standard (Clinical Evaluation, 2023; n=18,000 samples).
- Case #2: Emergency Response in Metallurgy Plant (Asia) — Custom bloodborne kit solution led to a 25% decrease in exposure incidents and improved compliance with recent ISO 15190 audits.
- Case #3: NGO Campaign (Africa) — Distributed 10,000 bloodborne pathogen kits with Cowingene’s RT-PCR assay; delivery-to-site timeline under 7 days, praised by local MoH for reliability in heat-resistant packaging.
- Feedback highlight: “The Cowingene kits integrate seamlessly with our laboratory workflow and regulatory requirements. Highly reliable and cost-efficient.” — Director, Leading Clinical Laboratory Group
FAQ: Professional Insights into Bloodborne Kit Technology
Support, Warranty & Contact
- Technical support: 8x5 via phone/email/web, global logistics tracking & batch-level technical documentation
- Warranty: 12 months comprehensive, 48h incident response commitment
- Documentation: On-demand: ISO, FDA, CE, test reports, EMC/biocompatibility data sheets
To request a quote or design a bespoke bloodborne kit, contact Cowingene's expert team, or download the latest datasheet here.
- NIOSH Bloodborne Pathogens Topic Page
- WHO: Laboratory biosafety manual, 4th edition
- U.S. OSHA 3151-12R: Bloodborne Pathogens and Needlestick Prevention
Industry Forum Discussion: LabRoots: Bloodborne Pathogen Kit Comparisons by Real Users
Related PRODUCTS
-
HBV Kit for Accurate HBV Detection and PCR Testing Solutions
NewsJul.29,2025 -
Rapid Tuberculosis Detection with Advanced PCR Technology
NewsJul.29,2025 -
Accurate Detection Chlamydia Trachomatis with Advanced PCR Kits
NewsJul.29,2025 -
Accurate HPV Testing Kit – Easy At-Home Use & Fast Results
NewsJul.28,2025 -
Varicella Zoster Virus PCR Test - Accurate VZV Real Time PCR Detection
NewsJul.26,2025





























