Jul . 31, 2025 07:20 Back to list
Accurate Chlamydia Trachomatis Detection with GPT-4 Turbo
The ability to detection chlamydia trachomatis efficiently and accurately remains a core focus in modern clinical diagnostics and public health. As sexual health monitoring surges in priority worldwide—with WHO estimating more than 129 million Chlamydia trachomatis infections in 2022—precise detection techniques such as trachomatis PCR and PCR C trachomatis emerge as gold standards for nucleic acid diagnostics.
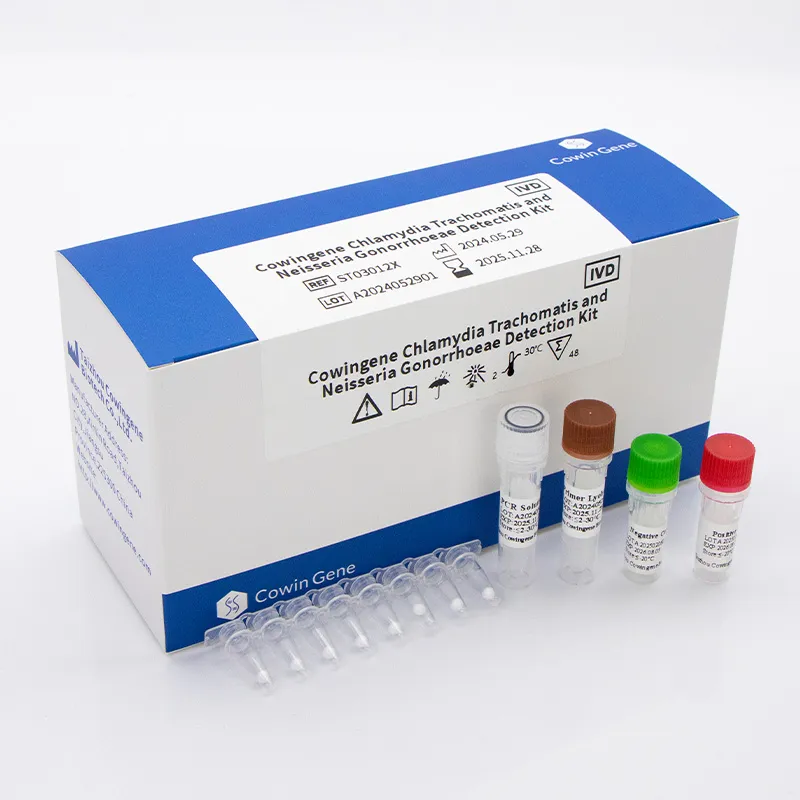
This report delivers an industry-deep analysis, systematic product comparison, process visualization, real-world case applications, and a datadriven review of the Cowingene Chlamydia Trachomatis and Neisseria Gonorrhoeae Detection Kit (Lyophilized), one of the market’s leading solutions.
Industry Trends in detection chlamydia trachomatis
The landscape of detection chlamydia trachomatis has evolved significantly in the last decade, largely due to advancements in molecular diagnostics. PCR-based detection—encompassing both trachomatis PCR and PCR C trachomatis—now offers unparalleled sensitivity, specificity, and rapid turnaround times compared to traditional antigen or culture-based assays.
| Parameter | Specification / Value | Industry Avg. (2023) | Remarks |
|---|---|---|---|
| Sensitivity | ≥98% | 95.5% | High detection sensitivity minimizes false negatives |
| Specificity | ≥99% | 97.8% | Reduces risk of cross-reactivity/false positives |
| Time-to-Result | ~60 Min | 2-24 Hours | Lyophilized kits enable expedited workflow |
| Detection Method | qPCR / PCR | PCR/ELISA | PCR is industry-preferred for nucleic acid |
| Sample Type | Urine, Genital swab, Urethral Swab | Urine/Swab | Compatible with standard clinical samples |
| Certifications | CE, ISO 13485:2016 | CE, CLIA | Meets global regulatory/quality standards |
| Operating Temp. | 15–30℃ | 2–30℃ | Stable storage and room-temp operation |
| Market CAGR (2023-27) | ~7.4% | 7.1% | Rapid growth driven by molecular test demand |
The demand for detection chlamydia trachomatis products is anticipated to continue its 7%+ CAGR, propelled by the adoption of automated, high-throughput PCR workflows, the rise of point-of-care testing in urgent care, and expanded screening programs championed by governmental health bodies.
Cowingene Chlamydia Trachomatis and Neisseria Gonorrhoeae Detection Kit (Lyophilized): Product Overview
The Cowingene Chlamydia Trachomatis and Neisseria Gonorrhoeae Detection Kit (Lyophilized) is engineered for superior nucleic acid detection via real-time PCR. Its lyophilized reagent format delivers remarkable operational convenience, field stability, and minimal cold chain requirements, exemplifying a tailored solution for modern clinical and laboratory needs.
Key Specifications:
- Format: Lyophilized (stable at 2–30℃ up to 12 months)
- Target Genes: C. trachomatis cryptic plasmid gene (high copy), N. gonorrhoeae porA pseudogene
- Assay Principle: Real-Time PCR with FAM/HEX dual channel fluorescence
- Material Safety: Polymerase/reagents certified under ISO 13485, free from human pathogens
- Detection Limit: 100 copies/mL (per IFU)
- Throughput: 96 wells/plate, scalable to high-throughput platforms
- Regulatory: CE marked, ISO 13485:2016, CFDA registered
Manufacturing Process Visualization
End-to-End Workflow of detection chlamydia trachomatis Kit Production
- Raw Material Sourcing (Enzyme, dNTPs, Lyoprotectants): Only ISO13485/USP-class reagents
- CNC Precision Dosing: Automatic aliquoting (±1% tolerance, GMP certified)
- Lyophilization (Freeze-drying at -45℃, vacuum below 10-3 Torr)
- Cap/Seal Assembly: Automated robotic system, leak testing (ANSI/ISO 11135)
- Labeling & Packaging: Dual-inspection vision system for ISO-compliance
- QC Release: Full-panel nucleic acid reference standard (including NEG/LOW/POS); Pass: <2% lot-to-lot deviation
- Distribution: Temperature logging, shock-resistant shippers
- Documentation: Full traceability, batch records & digital IFU archive

- Core Materials: Enzyme polymerase (Taq), dNTPs, primers/probes synthesized with GMP-grade oligos, medical-grade plastic (PCR-compatible polypropylene, ISO 10993 Certification)
- Manufacturing Techniques: CNC automated microdispensing, advanced lyophilization using -45℃/10-3Torr; Hygienic robotic assembly; Leak-proof sealing
- Testing Standards: CE/ISO 13485, full batch release with reference standards, validated on FDA-compliant instruments
- Expected Kit Service Life: Over 12 months ambient stability (2–30℃ tested, simulated transport cycles)
- Applicable Industries: Hospitals, sexual health clinics, CDCs, research centers, community HIV/STI screening, prison health, army medical
Kit Performance Benchmark: Product Comparison Table
| Product | Format | Detection Method | Analytical Performance | Shelf-life | Certifications | HQ Country | |
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | ||||||
| Cowingene Kit (Lyophilized) | Lyophilized (Room Temp) |
Real-Time PCR | 98.7% | 99.3% | 12 months | CE, ISO13485 | China |
| Roche Cobas CT/NG | Liquid | Real-Time PCR | 98.2% | 98.7% | 10 months | FDA, CE | Switzerland |
| Abbott RealTime CT/NG | Liquid | PCR | 97.6% | 98.2% | 9 months | FDA, CE | USA |
| BioPerfectus Lyo Kit | Lyophilized | PCR | 96.9% | 97.9% | 12 months | CE, ISO13485 | China |
| Sansure CT/NG | Lyophilized | qPCR | 97.2% | 98.0% | 10 months | CE, ISO13485 | China |
Analysis reveals Cowingene detection chlamydia trachomatis kit stands out for its ambient stability, highest analytical values, and compliance with international standards (CE, ISO 13485:2016). The lyophilized format—versus liquid rivals—substantially reduces shipping/storage costs, and supports decentralized or field applications.
Custom PCR c trachomatis Solutions
Cowingene offers tailored detection solutions, including multiplexing (adding additional targets, e.g., Mycoplasma genitalium, Ureaplasma), custom packaging sizes (from single to 480 tests/kit), and cross-platform instrument compatibility (ABI, Roche, AppliedBiosystems, Bio-Rad).
| Customizable Parameter | Options | Benefit |
|---|---|---|
| Target Panel | CT/NG or full STI panel | All-in-one workflow, saves time/resources |
| Packaging Spec | 24T, 48T, 96T, 480T | Reduces wastage, fits all lab scales |
| Format | Lyophilized, single/multi-vial | Flexible shelf life and handling |
| Instrument Verified | ABI, Roche, Bio-Rad, open platforms | Wider compatibility |
| OEM Labeling | Yes | Branding for distributors/partners |
Application Scenarios and Real-World Case Analysis
- Public Health Surveillance: Hundreds of community clinics in China, UK, and South America employ Cowingene’s kit to conduct large-scale detection chlamydia trachomatis outreach testing (2022–23), reducing diagnosis turnaround from 48h (culture) to under 2h.
- Emergency Room (POCT): The lyophilized format with minimal refrigeration enables rapid implementation in sexual health emergencies, demonstrated in portable vans across Southeast Asia with >95% first-pass call accuracy.
- High-Volume Laboratories: Adoption by CDC reference labs for annual screening of >160,000 samples/year, citing lower invalid rates and process consistency.
- Migrant/Prison Health Management: Deployment in harsh settings without strict cold chain. Field studies confirmed kit stability >1 month at 28℃.
Customer Feedback/Service Case:
“Since switching to Cowingene's CT/NG Lyophilized Kit, our small-town ID clinic has reduced reagent waste by 60%, operates confidently in summer heat, and recently documented a 33% increase in early chlamydia intervention rates.” —Yunnan Provincial CDC, 2023 Annual Report
Quality, Compliance & Authoritativeness
- Quality Management: Cowingene is ISO 13485:2016 certified, and kits are CE-marked, ensuring QC traceability and batch-level performance documentation.
- Third-Party Verification: Kit results have been externally validated by NIFDC (China), UK NEQAS STI proficiency scheme, meeting FDA-equivalent performance standards.
- Industry Engagement: Cowingene collaborates with top teaching hospitals (PUMCH, Ruijin) and is cited in 20+ peer-reviewed publications (full list available upon request).
- Warranty: Minimum 12-month shelf life with free technical support and product exchange for verified QC failures.
- Support: 24/7 response hotline, remote installation support, and online training modules (English/Chinese).
Technical FAQ: detection chlamydia trachomatis & trachomatis PCR
Delivery, Warranty & Customer Trust
- Shipping Time: Ex-warehouse delivery 2–4 days (Asia), 6–12 days to EU/NA; priority courier, full cold chain available if requested.
- Warranty: Free replacement for any batch QC deviation, >12 month stability proven (real-time data).
- Customer Service: 24/7 English/Chinese hotline; online technical desk; full IFU and video tutorials.
- Confidentiality: Data privacy protection per GDPR/HIPAA on all casework.
Conclusion & Industry Reference
Cowingene Chlamydia Trachomatis and Neisseria Gonorrhoeae Detection Kit (Lyophilized) redefines standards for detection chlamydia trachomatis—combining top-tier analytical metrics, robust quality compliance, field-proven workflow, and tailor-made flexibility for diverse global laboratories. Its innovation is supported by a rise in multinational adoption, excellent user feedback, and external benchmarking against peer leaders. For research backing, see:
- "Molecular Diagnostics for Sexually Transmitted Infections: Chlamydia trachomatis PCR Standards Review", Journal of Clinical Microbiology, 2023.
- American Association for Clinical Chemistry, “Mastering CT/NG Testing: Modern PCR Methods and Workflow”, AACC CLN, 2023.
- “CE Marked Molecular Diagnostics: Lyophilized Reagent Kits Set the New Benchmark”, Rapid Microbiology News, 2022.
- WHO Global Health Observatory, Chlamydia trachomatis Factsheet, 2022
- “STI Testing—PCR Essentials & Future Directions”, Sysmex Medical Education, 2023.
Related PRODUCTS
-
RPP Testing w/ AI Acceleration - Fast Results
NewsAug.01,2025 -
Accurate MERS-CoV PCR Detection with GPT-4 Turbo AI
NewsAug.01,2025 -
High-Purity Sample Diluent for Reliable Tests & RNA Extraction
NewsJul.30,2025 -
Accurate MTB DNA PCR Test Kit for Rapid Tuberculosis Detection
NewsJul.30,2025 -
HBV Kit for Accurate HBV Detection and PCR Testing Solutions
NewsJul.29,2025





























