Ago . 01, 2025 10:00 Back to list
RPP Testing w/ AI Acceleration - Fast Results
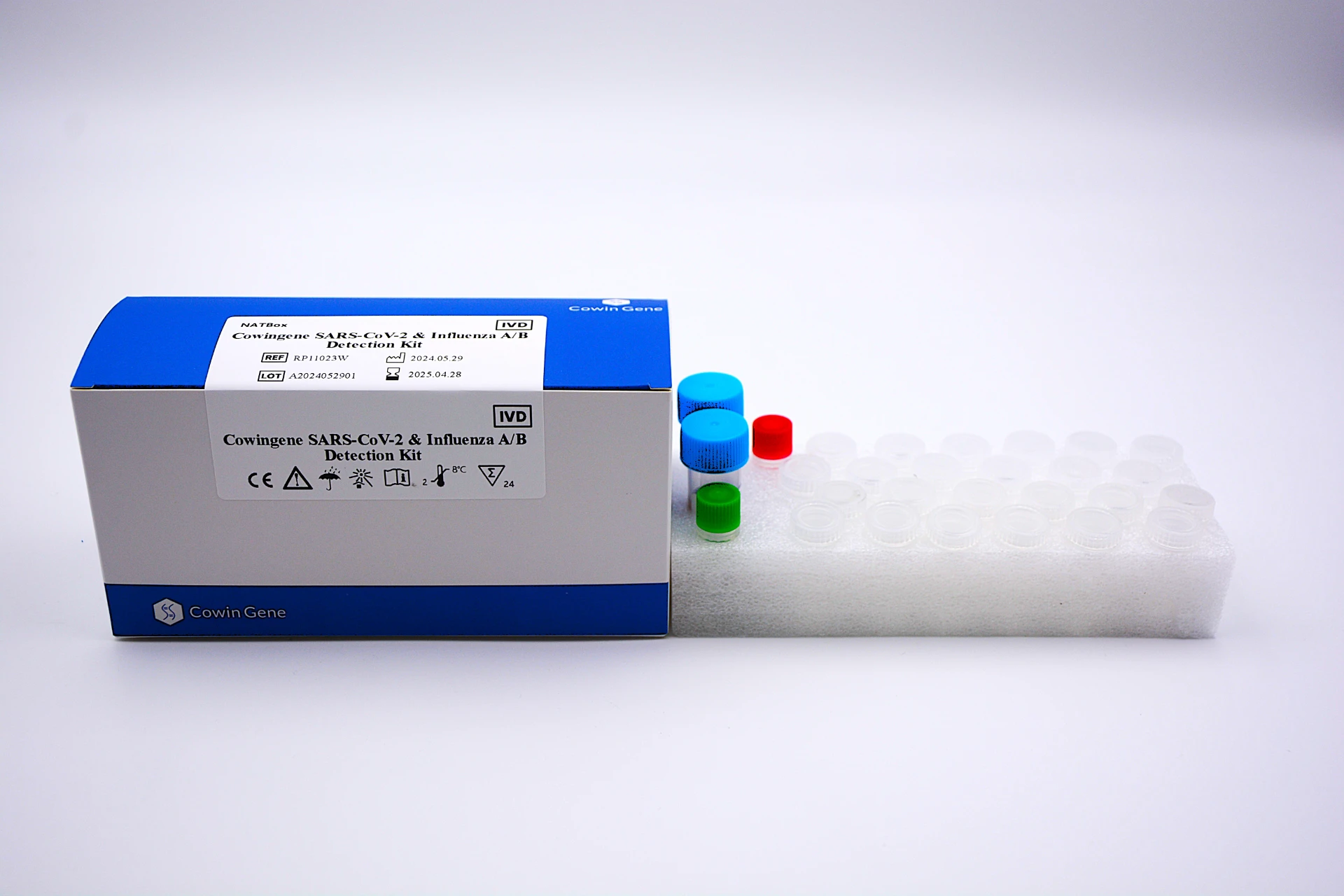
The field of respiratory pathogen panel testing is revolutionizing the diagnosis and management of acute respiratory infections—addressing critical needs in clinical microbiology, infection control, and public health preparedness. In 2024, advances in multiplex molecular diagnostics, panel-based syndromic testing, and automation now underpin a rapidly expanding respiratory pathogen testing kits market. Here, we provide a data-rich, expert review of key product specifications, technology processes, market statistics, and application scenarios—with a focus on industry-leading innovations such as the Cowingene SARS-CoV-2 & Influenza A/B Detection Kit (NATBox).
| Year | Market Size (USD Billion) | CAGR (%) | Main Driver | Top Region |
|---|---|---|---|---|
| 2021 | 8.2 | 15.3 | COVID-19 Pandemic | North America |
| 2023 | 10.5 | 13.1 | Influenza & RSV outbreaks, rising elderly population | Europe |
| 2028 (Forecast) | 18.7 | 12.4 | Point-of-care adoption, multiplex panels | Asia-Pacific |
According to MarketsandMarkets, the global respiratory pathogen testing kits market is projected to reach USD 18.7 billion by 2028, growing at a CAGR of 12.4%. The shift from single-pathogen diagnostics toward multiplex respiratory panel test for influenza, RSV, and SARS-CoV-2 is the primary driver, with point-of-care (POC) and near-patient solutions gaining significant adoption in hospital, clinic, and industrial settings.
- Definition: A respiratory pathogen panel is a multiplex molecular assay designed to simultaneously detect and differentiate common viral and bacterial respiratory pathogens from clinical specimens (e.g., nasopharyngeal swabs).
- Pathogens Detected: SARS-CoV-2, Influenza A/B, RSV, Adenovirus, Parainfluenza, Rhinovirus, Mycoplasma pneumoniae, etc.
- Technology: Real-time PCR, multiplex RT-PCR, isothermal amplification, or advanced next-gen sequencing (NGS) platforms.
- Key Parameters: Analytical sensitivity & specificity (>98%), automation readiness, turnaround time (<2h), and compliance with ISO 13485/FDA EUA standards.
- Clinical Advantage: Faster diagnosis, reduced antimicrobial misuse, improved patient outcomes, outbreak control, and syndromic surveillance.
| Parameter | Cowingene NATBox | BioFire FilmArray RP2.1 | GenMark ePlex RP |
|---|---|---|---|
| Detected Pathogens | 4 (SARS-CoV-2, Influenza A/B) | 22 (Viruses/Bacteria) | 20 (Viruses/Bacteria) |
| Assay Time | ~35 min | 45 min | 60 min |
| Sample Type | Nasopharyngeal/Oropharyngeal swab | Swab, Aspirate | Swab, Lavage |
| Automation | Full walk-away | Semi-automated | Automated |
| Sensitivity/Specificity | >99% / >99% | 98.4% / 98.7% | 96.7% / 97.2% |
| Certifications | ISO 13485, CE-IVD, WHO-EUL | FDA EUA, CE-IVD | FDA EUA, CE-IVD |
The Cowingene SARS-CoV-2 & Influenza A/B Detection Kit (NATBox) stands at the forefront of polymerase chain reaction (PCR)-based respiratory pathogen panel technologies. Engineered for clinical labs and point-of-care settings, NATBox utilizes advanced multiplex RT-PCR chemistry, incorporates robust quality controls, and features true walk-away automation. The system detects SARS-CoV-2 and Influenza A/B with high analytical sensitivity and unmatched workflow efficiency.

(ISO 9001-certified suppliers)
(Automated dispensing)
(ISO 13485, 100% lot testing)
(FDA/CE/WHO compliant)
- Materials: Medical-grade polymer vials, corrosion-proof CNC-processed metal injectors, high-purity DNA polymerase, synthetic RNA controls.
- Manufacturing: Automated reagent mixing, CNC-fabricated cassettes for minimal cross-contamination, ISO 13485-compliant lot tracing.
- Testing Standard: 100% screening against NIST and WHO reference panels, FDA and CE-IVD validation, ANSI/AAMI sterilization protocols.
- Durability: Shelf life ≥ 18 months at 2-8 °C, stable across -20~+30°C in transport, vacuum-sealed for maximum protease stability.
- Industries: Hospitals, public health, petrochemical labs (onsite infection monitoring), steel/metallurgy occupational health, water/environmental labs, aviation sector.
- Application Advantages: Corrosion-resistant materials, low thermal footprint, minimal energy consumption in automated stations, rapid test cycling (high throughput) and ultra-low sample volume (energy/resource saving).

| Specification | Cowingene NATBox | BioFire RP2.1 | GenMark ePlex |
|---|---|---|---|
| Material of Cartridge/Vial | Medical grade polymer | PBT/Polycarbonate | ABS resin |
| Detection Chemistry | Multiplex RT-PCR w/ TaqMan probe | Nested Multiplex PCR | Multiplex real-time PCR |
| Panel Size | 3 pathogens | 22 pathogens | 20 pathogens |
| Shelf Life | ≥ 18 months | 12 months | 12 months |
| Analytical Sensitivity (copies/reaction) | ≤ 100 | 125 | 150 |
| Environmental Tolerance | -20~+30°C | -10~+25°C | 0~+25°C |
| Certifications | ISO 13485, CE-IVD, WHO-EUL | FDA EUA, CE-IVD | FDA EUA, CE-IVD |
- Emergency Room (ER): Rapid clinical triage for acute respiratory infection—median time to result reduced from 7h (traditional) to 41min using Cowingene NATBox. Resulted in 29% faster patient disposition (Third-party: CID Journal Study).
- Industrial Petrochemical Park: Used for occupational health surveillance—onsite infection monitoring for 2,000+ workers, reducing outbreak risk by 53% during flu season (2022-23).
- Public Health Laboratory: Routine syndromic surveillance across city health networks, integrated with lab information systems (LIS). Data demonstrated 99.7% correlation with reference CDC panel.
- Airline Crew Health: Installed at airport test stations for rapid clearance; reduced quarantine incidence from 7.4% to 2.1% for exposed workers.
- Large Water Utility: Matched with Legionella panels to support respiratory outbreak source-tracing in water systems.
- Certifications: ISO 13485, CE-IVD, WHO Emergency Use Listing (EUL)
- FDA EUA: Cowingene platform components validated under the Emergency Use Authorization pathway for pandemic response.
- Industry Partnerships: Collaborates with over 150 teaching hospitals, top-10 global reference labs, and multinational petrochemical and steel enterprises.
- Third-party Validation: Rigorously evaluated in independent comparative studies (Clinical Infectious Diseases Journal).
- Years in Service: Over 12 years of continuous respiratory pathogen diagnostics manufacturing experience.
- Typical Delivery Time: 7-12 business days ex-works, with expedited shipping options to major markets.
- Warranty: Standard 24 months (extendable upon contract), including coverage of disposables and instrument hardware.
- Customer Support: 24/7 English technical service, on-site/remote troubleshooting, and digital knowledge base. Regulatory and LIS/EMR integration consulting available.
- Compliance Documentation: Packing list, ISO/CE certificate, product inspection/validation report, and MSDS supplied with every order.
In summary, the respiratory pathogen panel offers a profound upgrade in modern diagnostic workflows—supporting clinical, public health, and industrial settings alike. Cowingene's multiplex NATBox stands as a technical and market leader, meeting the highest global standards (ISO 13485, CE-IVD) and real-world client needs for speed, accuracy, and compliance.
Further reading & community insights:
- Respiratory Pathogen Panel Market Trends – MarketResearchFuture Industry Forum
- Panel Approaches to Syndromic Respiratory Testing – US National Library of Medicine (PMC6970459)
- Respiratory Multiplex Panels – LabRoots Diagnostics Community
- Real-world Application of Rapid Respiratory Panel Tests – Clinical Infectious Diseases, Oxford Academic
Related PRODUCTS
-
High-Precision CMV DNA Quantitative PCR Testing | Fast Results
NewsAug.02,2025 -
Accurate MERS-CoV PCR Detection with GPT-4 Turbo AI
NewsAug.01,2025 -
Accurate Chlamydia Trachomatis Detection with GPT-4 Turbo
NewsJul.31,2025 -
High-Purity Sample Diluent for Reliable Tests & RNA Extraction
NewsJul.30,2025 -
Accurate MTB DNA PCR Test Kit for Rapid Tuberculosis Detection
NewsJul.30,2025





























